THE CHEMICAL EFFECT OF ELECTRIC CURRENT- ELECTROLYSIS
When electric current passes through a current conducting solution/liquid (electrolyte). The electrolyte decomposes in to its ions .This process is known as electrolysis. This is the chemical effect of electric current.
EXPERIMENT
CuSO4 carbon rod
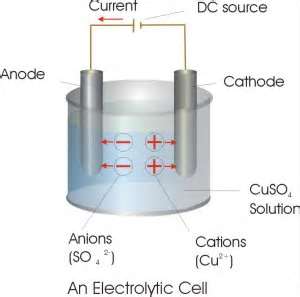
Take copper sulphate solution in a beaker. Dip two carbon electrodes in it. Connect these electrodes to a battery. By the passage of electricity the solution decomposes to its ions,ie Cu(2+) ions and SO4(2-) ions are formed. Positively charged copper ions moves towards negative electrode(Cathode). Negatively charged sulphate ions move towards positive electrode(Anode). Sulphate ions move towards anode and looses two electrons. The positively charged Copper ions move towards negative electrode gain two electrons and get deposited as copper atom. We can see this as shiny reddish deposit of Cu on the Cathode.
Why the color of CuSO4 Solution decreases?
Due to the decrease in number of Copper ions in the solution , the concentration of the solution decreases. So the blue Colour of CuSO4 decreases
Instead of Carbon cathode if we use copper electrodes is there any change in bluish color of solution?
No there is no change in color. The positively charged copper ions move towards cathode and accept two electrons from copper electrode.and deposited there. In the Anode the Cu atom loose two electrons reaches in the solution .So there is no change in concentration of electrolyte.Therefore no change in color.
APPLICATIONS OF ELECTROLYSIS
For electroplating
To purify metals
For anodisation




No comments:
Post a Comment